|
||
Molecular DynamicsSodium Chloride nanoparticles formed from sea salt are a major component of atmospheric aerosols, which in effect can directly impact the atmospheric radiative transfer or indirectly impact the climate by acting like cloud condensation nuclei. These crystal-like particles can best be understood by studying their solid-vapor and solution-vapor interfaces, which is done here with the use of molecular dynamics (MD) simulation techniques. MD simulations provide an excellent tool to investigate the initial stages of hygroscopic growth of nanoparticles that once the use of deliquescence relative humidity (DRH) had been able to characterize for particles less than 10 nm. Understanding the interfacial profiles and the significant effect of size on the hygroscopic properties of nanoparticles will assist in our understanding of the impacts that the aqueous layer of these sea salt aerosols can have on atmospheric chemistry. This could include a site for atmospheric pollutants such as nitric acid and nitrous oxides, as well as a source of chlorine which can affect the ozone balance and oxidize organic substances. Peer-reviewed publications on this topic:
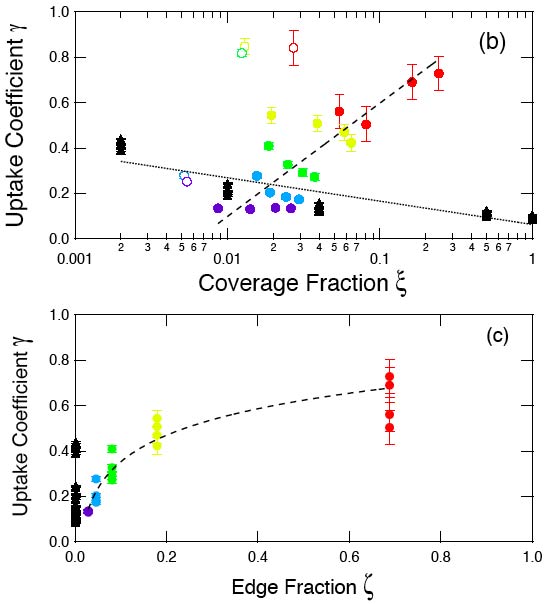
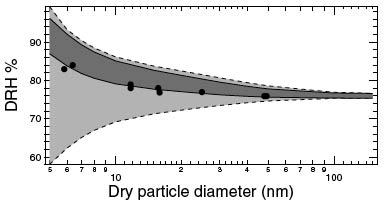
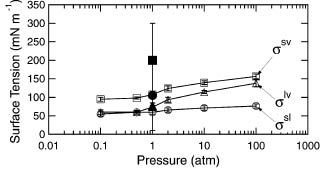
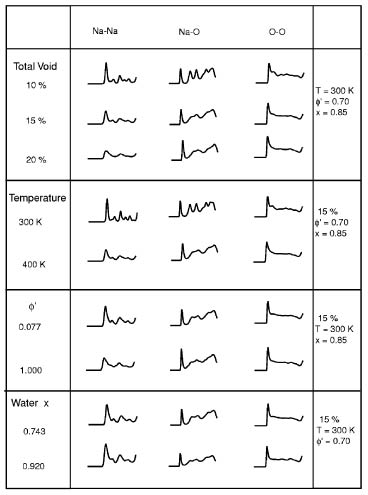
A study of deliquescence properties using MD simulations of NaCl nanparticles smaller than 100nm revealed that the DRH increases with decreasing particle diameter. Planar uptake coefficients and particle uptake coefficients showed a linear dependence on the logarithm of the coverage fraction and the edge fraction respectively. This paper introduces newly calculated Tolman length and surface tension estimates of solid-liquid and solid-vapor interfaces in NaCl-water-air systems using classical MD simulations. The correction values to surface tension greatly reduce the uncertainty that produces the over-prediction discrepancy of DRH with decreasing particle size (<10nm). Research on surface tensions in NaCl-Water-Air Systems from MD simulations contribute to reduced uncertainty in bounding values for solid interfaces with the use of energy-integral and test area methods. In addition, upper bound values calculated from the thermodynamic relation for energy difference between interface and bulk phases show a weakly positive correlation with pressure for all the interfaces. Classical MD simulations were used in this study to observe the dissolution at the NaCl-water interface by introducing defects to the crystal surface and in the crystal volume, or increasing the 'void fraction'. The dissolution of NaCl was found to have a strong dependence on void fraction, and increasing the temperature was also found to decrease the critical void fraction. |

|
|